MULTICORR: Galvanic Corrosion in Multi-material Joints
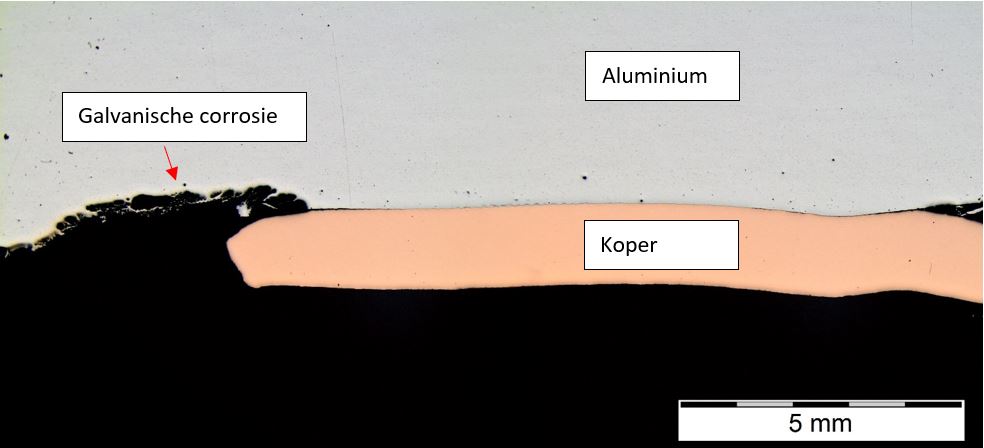
Galvanic corrosion by contact between aluminium and copper after 6 weeks of salt spray testing
Different solutions are available to prevent galvanic corrosion, but depending on the application and specific requirements with regard to the components, not all solutions are functional.
Project goals
This project aims to investigate the risk of galvanic corrosion in combinations of different materials (mainly metals). The project was started in 2019 with an extensive user group that is situated in various sectors: companies in the transport sector and in metal construction and manufacturing companies that produce appliances or utensils for aggressive environments (for example, offshore).
Concrete interest mainly consists of bolt and other screw connections, with specific focus on the following metals:
- Galvanized steel (zinc and zinc-aluminum-magnesium galvanization)
- Other protection methods with zinc: zinc flake coatings, zinc paints
- Stainless steel
- Aluminium
In addition, research will also be conducted into the risk of galvanic corrosion when joining metals with carbon fiber reinforced composites.
A few examples that can be investigated:
- Contact of copper and aluminium in batteries or heat exchangers
- Bolted connections
- Contact of steel and aluminium for lightweight applications (for example, the transport sector)
- Black and white joints (steel with stainless steel)
- Contact of metals with composites for lightweight applications (for example, the transport sector)
The research questions for such cases can include:
- Is there a risk of galvanic corrosion in my application? Which effect does galvanic corrosion have on the life cycle of systems?
- Which codes of good practice are there already to prevent galvanic corrosion for my application?
- Which techniques are available for my application to prevent galvanic corrosion and which technique is the most suitable for the life cycle of my component?
Project description
The studies included translation research of available information in publications, short corrosion tests (polarization tests), cyclic corrosion tests and atmospheric tests. An example of test sample with a galvanized part (ZM 310) in combination with galvanized bolts and stainless steel bolts is shown in the Figure below. These lab tests are combined with simulations to predict galvanic corrosion.
In a first phase, current applications at companies are examined for their risk of galvanic corrosion. Protection methods to prevent galvanic corrosion in these applications are then also tested.
Test sample: different bolted connections are tested on a galvanized part (ZM310 galvanization)
Is galvanic corrosion a problem in your company or do you have questions about this theme? Please fill in your details below!
Results
The project ended in spring 2022. In total, more than 20 material combinations were tested in various environments. Mainly combinations between galvanised steel, stainless steel and aluminium were investigated. The collective research included electrochemical polarisation tests, cyclic corrosion tests and atmospheric exposure tests and an investigation into applicability of simulation in predicting galvanic corrosion. It was demonstrated that these models are suitable for providing insight into which materials will corrode first.
The collective research and the application-oriented research show that galvanic corrosion strongly depends on the material combination and the environment. Transport environments in particular pose risks given the use of road salt.
For the combinations between galvanised steel, stainless steel and aluminium, it can be concluded that:
- Stainless steel causes galvanic corrosion on galvanised steel, but the acceleration factor is limited.
- Stainless steel causes galvanic corrosion on aluminium, which may be more severe, given that it takes a localised form.
- Galvanised steel and aluminium are compatible and do not cause galvanic corrosion. However, when the zinc layer is consumed, the steel will cause severe galvanic corrosion on the aluminium.
For the tests carried out, it can be concluded that:
- Polarisation tests (galvanic series) show the mutual proportions of noble and base. These tests provide no insight into expected local corrosion phenomena.
- The cyclic corrosion test (VDA 233-102) shows correlation with real environment in the observed phenomena: local corrosion of aluminium and accelerated corrosion of zinc in contact with stainless steel. This correlation was best for the most aggressive atmospheric environment. For the less aggressive environments, the correlation could not yet be made, as corrosion is slow in these environments. Longer test durations are needed to investigate this further.
- The atmospheric exposure tests give a 1-to-1 picture of phenomena to be expected in a given environment. However, these tests require time, especially in environments that are not very aggressive.